Are you preparing for CBSE Class 10 Science exams and looking for case study-based questions from Chapter 2 – Acids, Bases and Salts?
Here, we provide high-quality CBSE Class 10 Chemistry case study questions with detailed answers in quiz format to make your preparation more interactive and exam-focused.
📘 Chapter Details
- Chapter Name: Acids, Bases and Salts
- Subject: Science – Chemistry
- Class: 10 (CBSE Board)
- Exam: CBSE 2025 Board Exam
- Resource Type: Case Study Based Questions with Solutions (Quiz Format)
🔍 What are Case Study Based Questions?
Case study questions are designed to test your understanding of concepts in real-life situations. For Acids, Bases and Salts, these questions often include:
- pH value interpretation
- Uses and properties of acids and bases
- Neutralization reactions
- Applications of salts in daily life
These questions carry high weightage in CBSE Class 10 Board Exams, so practicing them is a must!

📂 Topics Covered in this Post
✔ Properties of Acids and Bases
✔ pH Scale and its Applications
✔ Chemical Reactions of Acids and Bases
✔ Uses of Salts in Everyday Life
✔ Identification of Acids, Bases, and Salts in Laboratory
📚 Case Study 1: pH Scale and Universal Indicator
The pH scale measures how acidic or basic a substance is by making use of hydrogen ion concentrations in them. The table below shows the pH values of five different substances and the color produced by a universal indicator.
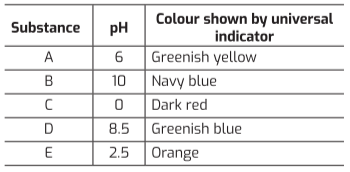
Based on the above information, answer the following questions:
Q1. Which of the following is/are true about substance B (pH 10)?
Substance B has a pH of 10, the highest among the options, making it the strongest base (Statement I is true). Mild bases like it are used in antacids (Statement II is true). Bases turn red litmus blue but have no effect on blue litmus (Statement III is false).
Q2. What happens when a solution of substance D (pH 8.5) is mixed with a solution of substance E (pH 2.5) in a test tube?
Substance D is a base and substance E is an acid. When they react, a neutralization reaction occurs, forming salt and water. This type of reaction is exothermic, releasing heat and increasing the solution’s temperature.
Q3. Arrange the substances A (pH 6), C (pH 0), and E (pH 2.5) in increasing order of their acidic strength.
Acidic strength increases as the pH value decreases. The pH values are A=6, E=2.5, and C=0. Therefore, the correct increasing order of acidic strength is A < E < C.
Q4. Equal volumes of hydrochloric acid and sodium hydroxide solutions of the same concentration are mixed. What colour would a pH paper show in the resulting solution?
A strong acid (HCl) and a strong base (NaOH) neutralize each other to form a neutral salt (NaCl) and water, resulting in a pH of 7. A universal indicator or pH paper shows a yellowish-green color at pH 7.
Q5. Which row in the table from the case study has incorrect information?
Phenolphthalein remains colorless in acidic solutions and turns pink in basic solutions. The information provided in this row is potentially misleading or contains a subtle error, making it the incorrect one.
📚 Case Study 2: Identifying Solutions
A teacher divided students into three groups and gave them various solutions to test their pH and
classify them.
Group A: Lemon juice, vinegar, colourless aerated drink.
Group B: Tomato juice, coffee, ginger juice.
Group C: Sodium hydroxide, sodium chloride, lime water.
Based on this, answer the following questions:
Q6. Which group(s) are likely to have a pH value (i) less than 7, and (ii) greater than 7?
(i) Less than 7 (Acidic): Group A and Group B. Solutions like lemon juice, vinegar, tomato juice, and coffee are acidic.
(ii) Greater than 7 (Basic): Group C. Sodium hydroxide and lime water are basic. Sodium chloride is neutral (pH=7).
Q7. List two ways of determining the pH of a solution.
Two common ways to determine pH are:
1. Using pH paper or litmus paper.
2. Using a universal indicator solution.
Q8. Explain why sour substances are effective in cleaning tarnished copper vessels. OR Justify the statement “pH has great importance in our daily life” with two examples.
Cleaning Copper Vessels: Copper forms a green layer of basic copper carbonate when it tarnishes. Sour substances contain acids (e.g., citric acid in lemon) which neutralize this basic layer, forming a soluble salt that washes away easily, restoring the copper’s shine.
OR
Importance of pH in Daily Life:
1. Tooth Decay: The pH in our mouth can drop below 5.5 due to acid-producing bacteria, which causes tooth enamel to decay. Basic toothpastes neutralize this acid.
2. Body Function: Our body functions best within a narrow pH range of 7.0 to 7.8.
📚 Case Study 3: The Salt Story
Salt pans are used to produce salt from seawater by evaporation. This salt is a raw material for various sodium compounds, like sodium hydrogen carbonate. The table shows the composition of solids obtained from evaporating 1 litre of seawater.
Based on this, answer the following questions:
Q9. Which compound in the table reacts with acids to release carbon dioxide?
Calcium carbonate (CaCO3) is a metal carbonate, which reacts with acids to produce salt, water, and carbon dioxide gas.
Q10. How many grams of magnesium sulphate are present in 135 g of the solid evaporated from seawater?
According to the table, 45 g of solid contains 6 g of magnesium sulphate. Therefore, 135 g of solid will contain (6 / 45) × 135 = 18 g.
Q11. What is a saturated solution of Sodium Chloride called?
Brine is the common name for a concentrated aqueous solution of sodium chloride (NaCl).
Q12. What is the approximate pH of the acid used in the formation of common salt?
Common salt (NaCl) is formed from hydrochloric acid (HCl), a strong acid, and sodium hydroxide (NaOH), a strong base. Strong acids like HCl have a very low pH, typically between 1 and 3.
📚 Case Study 4: A Crispy Secret
Mrs. Tomar uses a compound of sodium ‘X’ to make pakoras crispy. It is a mild non-corrosive basic salt, also used as an ingredient in antacids. It is produced using sodium chloride as one of the raw materials.
Based on this, answer the following questions:
Q13. Identify the compound of sodium ‘X’.
Compound ‘X’ is sodium hydrogen carbonate (NaHCO3), also known as baking soda.
Q14. Is the pH value of the ‘X’ solution lower or higher than 7?
The pH value of a baking soda solution is higher than 7, as it is a mild basic salt.
Q15. Write the chemical equation for the preparation of ‘X’.
The chemical equation for its preparation is: NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
Q16. Write the chemical reaction that occurs when ‘X’ is heated.
When heated, baking soda decomposes: 2NaHCO3(s) → Na2CO3(s) + H2O(l) + CO2(g)
Q17. How would you test for the gas evolved on heating ‘X’?
The evolved gas is CO2. To test for it, pass the gas through lime water (calcium hydroxide solution). It will turn the lime water milky due to the formation of insoluble calcium carbonate.
📚 Case Study 5: A Medical Marvel
A girl fractured her leg in an accident. A doctor mixed a white powder with water and applied it over the fractured area. It soon turned into a white, solid, hard mass, creating a supportive cast for the bone to heal correctly.
Based on this, answer the following questions:
Q18. What are the common names for the ‘white powder’ and the ‘white hard solid mass’?
The ‘white powder’ is known as Plaster of Paris, and the ‘white hard solid mass’ is Gypsum.
Q19. What are the chemical names of the ‘white powder’ and the ‘white hard solid mass’?
The chemical name for Plaster of Paris is calcium sulphate hemihydrate (CaSO4·½H2O). The chemical name for Gypsum is calcium sulphate dihydrate (CaSO4·2H2O).
Q20. Why must the white powder be stored in a moisture-proof container?
Plaster of Paris must be stored in a moisture-proof container because it absorbs water from the air and hardens into gypsum, which makes it unusable.
Q21. Write the chemical equation for the reaction between the white powder and water.
CaSO4·½H2O + 1½H2O → CaSO4·2H2O
Q22. What is the difference in the number of water molecules between the white hard solid mass and the white powder?
The difference is between the water molecules in Gypsum (2) and Plaster of Paris (½). Difference = 2 – ½ = 1½ molecules.
📚 Case Study 6: The Nature of Acids
The acidic behaviour of acids is due to the presence of hydrogen (H+) ions, which they produce in water. Water, being a polar solvent, helps weaken the bonds in acid molecules, allowing them to ionize. Dry HCl gas, for instance, does not show acidic properties because ionization does not occur without water.
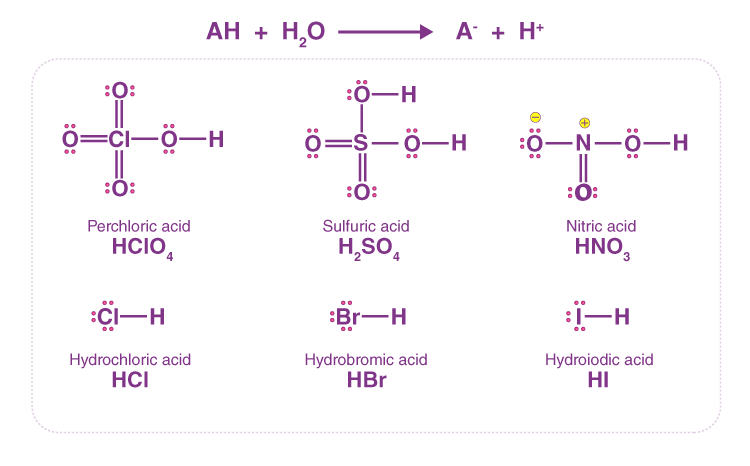
Answer the following questions:
Q23. Identify the wrong statement.
An increase in pH from 7 to 14 indicates an increase in basicity, which corresponds to a decrease in H+ ion concentration.
Q24. If the pH of a solution is 8, what is its [H+] ion concentration?
The concentration of H+ ions is calculated as 10-pH. Therefore, if pH = 8, the concentration is 10-8 M.
Q25. In terms of acidic strength, which one of the following is in the correct increasing order?
Water is neutral (pH~7), acetic acid is a weak acid, and hydrochloric acid is a strong acid. Therefore, the acidic strength increases in that order.
Q26. Which of the following compounds does not give H+ ions in an aqueous solution?
Ethanol (C2H5OH) is a covalent compound (an alcohol) and does not ionize in water to produce H+ ions. The other options are acids.
Q27. Four solutions P, Q, R, and S have pH values 1, 9, 3, and 13 respectively. Which statement is incorrect?
Solution P (pH 1) is acidic and turns blue litmus red. Solution Q (pH 9) is basic and turns red litmus blue. The statement that both turn red litmus blue is incorrect.
📚 Case Study 7: Sodium Compounds
A white powder, compound X of sodium, is a part of baking powder and is also used in antacids. When heated, it forms compound Y. When Y is dissolved in water, it forms a strong base and a weak acid, Z.

Answer the following questions:
Q28. What is compound X?
Compound X is sodium hydrogen carbonate (baking soda), which is used in baking powder and antacids.
Q29. What is compound Y?
When baking soda (NaHCO3) is heated, it decomposes to form sodium carbonate (Na2CO3), which is compound Y.
Q30. What is the nature of the solution formed by dissolving Y in water?
Sodium carbonate is a salt of a strong base (NaOH) and a weak acid (H2CO3). Its aqueous solution is alkaline due to hydrolysis.
Q31. Identify the compound, Z.
When Na2CO3 dissolves in water, it hydrolyzes to form NaOH (a strong base) and H2CO3 (carbonic acid), which is the weak acid Z.
Q32. Sodium carbonate is a basic compound because it is a salt of a:
Sodium carbonate (Na2CO3) is formed from the strong base NaOH and the weak acid H2CO3, making its solution basic.
📚 Case Study 8: Purification of Sodium Chloride
Sodium chloride from sea water contains impurities like chlorides and sulphates of calcium and magnesium, which are undesirable as they absorb moisture (deliquescent nature). To purify it, the salt is dissolved in minimal water, filtered, and then hydrogen chloride gas is passed through the saturated solution. Crystals of pure NaCl separate out, while soluble impurities remain behind.
Answer the following questions:
Q33. Select the correct statement regarding salt NaCl.
Pure sodium chloride is not hygroscopic (does not absorb moisture). The clumping of table salt is due to impurities like magnesium chloride.
Q34. What is the nature of an aqueous solution of common salt?
Common salt (NaCl) is a salt of a strong acid (HCl) and a strong base (NaOH). Therefore, its aqueous solution is neutral, with a pH of approximately 7.
Q35. In the reaction NaCl + H2O + CO2 + NH3 → X + Y, what are Y and X?
This is the first step of the Solvay process. The products are sodium hydrogen carbonate (X = NaHCO3) and ammonium chloride (Y = NH4Cl).
Q36. Which of the following compounds is alkaline in an aqueous medium?
Sodium carbonate (Na2CO3) is a salt of a weak acid and a strong base, so its solution is alkaline due to hydrolysis.
Q37. Which statement about NaCl is incorrect?
Statement (I) is incorrect. The chlor-alkali process uses a brine solution (NaCl) to produce sodium hydroxide (NaOH), chlorine, and hydrogen. It does not produce NaCl. Statements II, III, and IV (neutral salt with pH 7) are correct.
📚 Case Study 9: Plaster of Paris (POP)
Chemically, Plaster of Paris (POP) is calcium sulphate hemihydrate (CaSO4·½H2O). The name was given because it was first made from gypsum, which was primarily found in Paris. The half molecule of water of crystallisation means one water molecule is shared by two formula units of CaSO4.

Answer the following questions:
Q38. What is the difference in the number of water molecules between gypsum and Plaster of Paris?
Gypsum is CaSO4·2H2O and POP is CaSO4·½H2O. The difference is 2 – ½ = 3/2.
Q39. Plaster of Paris hardens by:
Plaster of Paris hardens by absorbing water (hydration) to form solid gypsum.
Q40. Which of the following statements is incorrect?
Dead burnt plaster is anhydrous calcium sulphate (CaSO4), formed when gypsum is heated to high temperatures, losing all its water of crystallisation.
Q41. Select the incorrect statement with respect to gypsum.
The chemical formula for gypsum is CaSO4·2H2O (calcium sulphate dihydrate). CaSO4·½H2O is the formula for Plaster of Paris.
Q42. Plaster of Paris is obtained by:
Plaster of Paris is prepared by carefully heating gypsum to a temperature of 373 K (100°C) to partially remove its water of crystallisation.
📚 Case Study 10: pH in Daily Life
pH is crucial in many aspects of daily life, from agriculture to self-defense in animals. Plants require a specific soil pH for growth, which can be adjusted using lime (for acidic soil) or organic manure (for basic soil). Bee stings are acidic and can be neutralized by a mild base like baking soda.
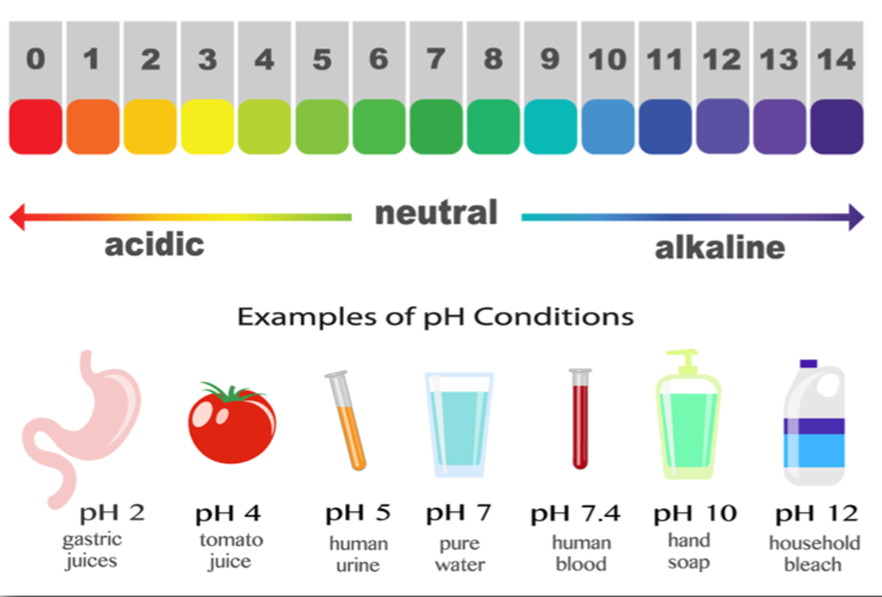
Answer the following questions:
Q43. When black copper oxide placed in a beaker is treated with dilute HCl, its colour changes to:
Copper(II) oxide (black) reacts with hydrochloric acid to form copper(II) chloride (CuCl2) and water. The resulting CuCl2 solution is bluish-green.
Q44. P is an aqueous solution of an acid and Q is an aqueous solution of a base. When these two are diluted separately, then:
Diluting an acid decreases its H+ concentration, so its pH increases (moves towards 7). Diluting a base decreases its OH– concentration, so its pH decreases (moves towards 7).
Q45. Which of the following acids is present in a bee sting?
Bee and ant stings contain methanoic acid, which is commonly known as formic acid.
Q46. A soap can cure an ant sting because:
Soap is a mild base (containing sodium hydroxide or potassium hydroxide). It neutralizes the formic acid from the ant sting, providing relief.
Q47. The pH of soil X is 7.5 while that of soil Y is 4.5. Which of the two soils should be treated with powdered chalk to adjust its pH?
Soil Y has a pH of 4.5, making it acidic. Powdered chalk is calcium carbonate (CaCO3), a base, which can be added to neutralize the acidity of the soil.
📚 Case Study 11: Baking Powder
Baking powder, used to make batter spongy, produces carbon dioxide on heating. It is a mixture of baking soda (NaHCO3) and a mild edible acid like tartaric acid. This acid neutralizes the sodium carbonate formed on heating baking soda, preventing a bitter taste.

Answer the following questions:
Q48. On passing excess CO2 gas in an aqueous solution of sodium carbonate, the substance obtained is:
The reaction is Na2CO3 + H2O + CO2 → 2NaHCO3. Sodium hydrogen carbonate is formed.
Q49. When sodium hydrogen carbonate is added to acetic acid, a gas evolves. Which statements are true about the gas? (I) It turns lime water milky. (II) It extinguishes a burning splinter. (III) It dissolves in a solution of sodium hydroxide. (IV) It has a pungent odour.
The evolved gas is CO2. It turns lime water milky, extinguishes flames, and reacts with NaOH. It is an odourless gas, so (IV) is false.
Q50. Select the correct statement regarding sodium hydrogen carbonate.
Sodium hydrogen carbonate reacts with acid in a soda-acid fire extinguisher to rapidly produce CO2 gas, which smothers the fire.
Q51. Acetic acid was added to a solid X. A colourless and odourless gas evolved, which turned lime water milky. Solid X is:
The gas is CO2. Acids react with bicarbonates (like sodium bicarbonate) to produce CO2.
Q52. Which of the following statements are correct regarding baking soda? (I) It is sodium hydrogen carbonate. (II) On heating, it gives sodium carbonate. (III) It is used to manufacture soap. (IV) It is an ingredient of baking powder.
Baking soda is not used in the manufacture of soap (that is washing soda). Statements I, II, and IV are correct.
📚 Case Study 12: Bleaching Powder
Bleaching powder (CaOCl2), or chloride of lime, is a yellowish-white solid with a strong smell of chlorine. It is formed when chlorine gas reacts with calcium hydroxide (slaked lime). It is used to bleach materials by releasing nascent oxygen when it reacts with dilute acid.

Answer the following questions:
Q53. Bleaching powder is used as:
Bleaching powder has many uses, including bleaching fabrics, disinfecting water, and acting as an oxidizing agent.
Q54. Bleaching powder is also known as:
All these names are used to refer to bleaching powder.
Q55. Bleaching powder gives a smell of chlorine because it:
Bleaching powder slowly reacts with carbon dioxide from the atmosphere to produce calcium carbonate and chlorine gas, which causes the smell.
Q56. Select the correct statement(s) regarding bleaching powder.
All the given statements about bleaching powder are correct.
Q57. Identify the product ‘X’ in the given reaction: Ca(OH)2 + Cl2 → X + H2O
This is the equation for the preparation of bleaching powder (calcium oxychloride).
📚 Case Study 13: Washing Soda
Washing soda is prepared via the Solvay process, which involves reacting brine with ammonia and carbon dioxide to form sodium hydrogen carbonate, which is then heated to produce soda ash (anhydrous sodium carbonate). Finally, recrystallisation of soda ash yields washing soda (Na2CO3·10H2O).
Answer the following questions:
Q58. Which of the following is a use of washing soda? (I) Removing permanent hardness of water. (II) Used in the glass industry. (III) Used in the paper industry. (IV) Manufacture of borax.
All four statements correctly describe the uses of washing soda.
Q59. What products are formed with water when sodium carbonate reacts with dilute hydrochloric acid?
The reaction is: Na2CO3 + 2HCl → 2NaCl + H2O + CO2. The products are sodium chloride and carbon dioxide.
Q60. Chief raw materials for the manufacture of washing soda are:
The Solvay process uses brine (sodium chloride), ammonia, and limestone (which provides CO2) as the main raw materials.
Q61. What is the action of sodium carbonate on litmus paper?
An aqueous solution of sodium carbonate is alkaline, so it will turn red litmus paper blue.
Q62. What products will be obtained when a solution of sodium carbonate and slaked lime is heated?
The reaction is: Na2CO3 + Ca(OH)2 → CaCO3(s) + 2NaOH(aq). Calcium carbonate precipitates out, leaving sodium hydroxide in solution.
📚 Case Study 14: Indicators
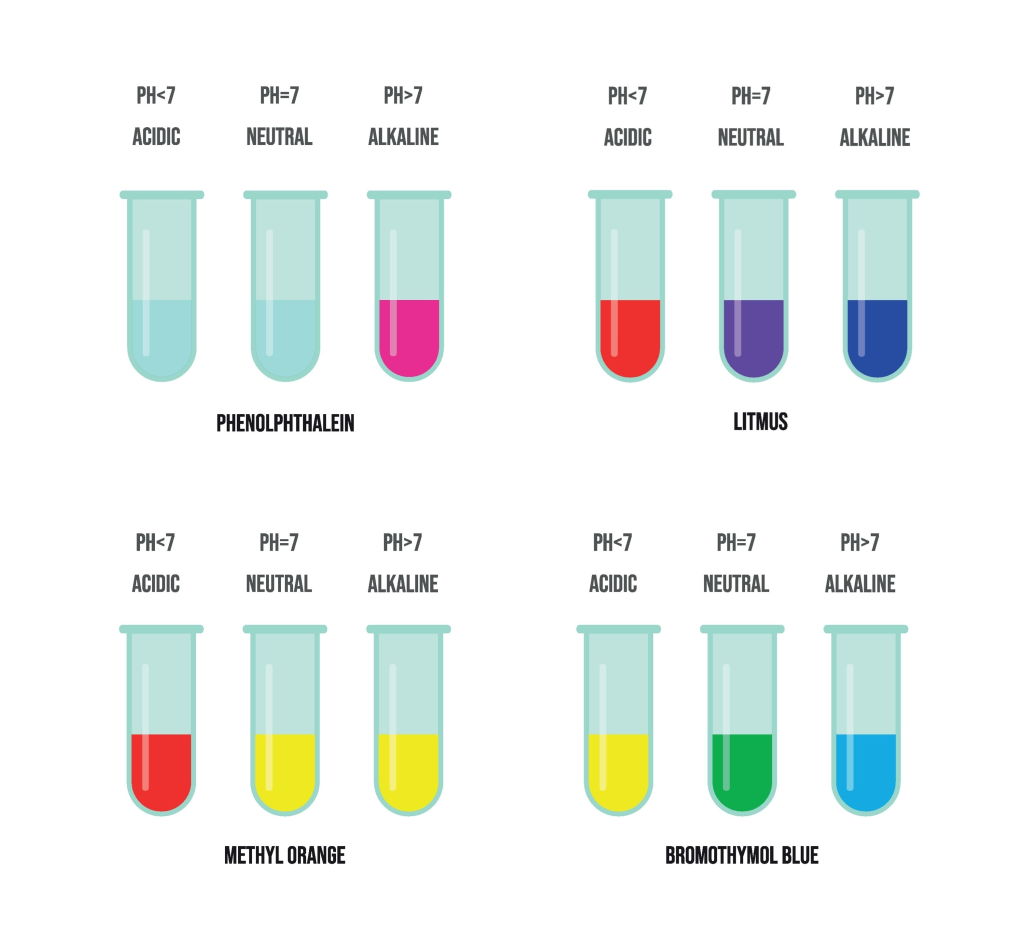
An indicator is a chemical compound added to a solution to detect its acidic or basic nature. Indicators are typically coloured organic substances that have one colour in an acidic medium and a different colour in an alkaline medium. Some indicators, called olfactory indicators, have different smells in acidic and basic media.
Answer the following questions:
Q63. Which one of the following will turn red litmus blue?
Baking soda solution is basic and will turn red litmus blue. The other options are acidic.
Q64. A solution turns blue litmus red. The pH of the solution is probably:
If a solution turns blue litmus red, it is acidic, meaning its pH is less than 7.
Q65. Solution ‘A’ turns red litmus blue. Solution ‘B’ turns blue litmus red and evolves CO2 with sodium carbonate. Identify ‘A’ and ‘B’.
‘A’ turns red litmus blue, so it is a base. ‘B’ turns blue litmus red and reacts with a carbonate to produce CO2, so it is an acid.
Q66. Select the incorrect option.
The flower of the hydrangea plant is typically blue in acidic soil and pink or red in basic soil. The provided colours are incorrect.
Q67. Which of the following can be used as an acid-base indicator by a visually impaired student?
Vanilla essence is an olfactory indicator; its smell changes in acidic and basic media, allowing it to be used by visually impaired students.
📚 Case Study 15: Indicator Observations
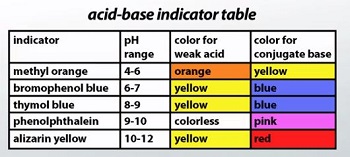
Acids turn blue litmus red, and bases turn red litmus blue. In phenolphthalein, acids are colourless while bases turn pink. In methyl orange, acids turn red/pink while bases turn yellow. These properties allow us to identify unknown substances.
Answer the following questions:
Q68. Which of the following substances does NOT turn red litmus solution to blue?
Phosphoric acid (H3PO4) is an acid; it will turn blue litmus red, not red litmus blue. The other options are bases.
Q69. Phenolphthalein’s colour in basic medium is ______, but in acid it is ______.
Phenolphthalein is a common indicator which is pink in basic solutions and colourless in acidic solutions.
Q70. Which of the following acids are edible? (I) Citric acid (II) Tartaric acid (III) Hydrochloric acid (IV) Carbonic acid
Citric acid (in citrus fruits), tartaric acid (in tamarind), and carbonic acid (in soft drinks) are edible. Hydrochloric acid is a strong mineral acid and is not edible.
Q71. The colour of methyl orange in a neutral solution is:
In its neutral state, methyl orange indicator has an orange colour.
Q72. Which of the following cannot act as an indicator?
Methyl chloride is a compound that does not change colour with pH and therefore cannot be used as an indicator. The others are all common acid-base indicators.

